Test for FGFR genetic alterations now
Fibroblast growth factor receptors (FGFRs)
- FGFRs are a family of receptors that promote gene expression related to cell proliferation, differentiation, migration, and angiogenesis1,2
- Genetic alterations in FGFRs may lead to constitutive signaling, promoting tumor growth and metastasis in several malignancies2-4









Expert opinion:
Why it is important to test early for FGFR genetic alterations
Your patients’ tumors may harbor actionable alterations
To find clinical trials for tumors with FGFR genetic alterations, as well as those for other actionable targets you may identify genomic analysis, go to ClinicalTrials.gov.
FGFR genetic alterations may initiate and negatively affect the course of some malignancies
ALTERED FGFR SIGNALING MAY BE A DRIVER EVENT IN ONCOGENESIS1,5
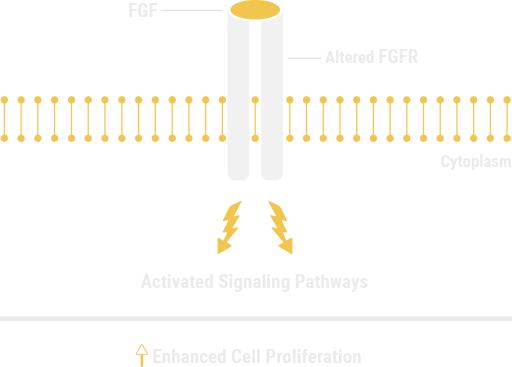

FGFR genetic alterations have been identified at varying frequencies across a variety of cancers6*
 Frequency of FGFR Alterations in a selection of solid tumors6
Frequency of FGFR Alterations in a selection of solid tumors6
| Cancer type | Frequency |
|---|---|
| Urothelial | 31.7% |
| Breast | 17.4% |
| Endometrial | 11.3% |
| Ovarian | 8.6% |
| Carcinoma of unknown primary† | 8.2% |
| Glioma | 7.6% |
| Cholangiocarcinoma | 7.0% |
| Gastric/gastroesophageal junction | 6.7% |
| Non–small cell lung | 5.2% |
| Pancreatic | 4.7% |
| Renal cell | 4.6% |
| Head and neck, squamous cell | 4.6% |
| Colorectal | 4.4% |
| Sarcomas | 4.0% |
| Neuroendocrine | 3.7% |
Alterations in specific FGFR expression may be related to prognosis or sensitivity to certain cancer treatments.4
* FGFR inhibitors are only approved for use in a limited number of tumor types. Some patients may be eligible for clinical trials.
†Origin of metastatic tumor cannot be identified.
Identification of actionable FGFR alterations may allow for utilization of biomarker-guided therapy7
The goal of precision medicine—targeting the right treatment for the right patient at the right time8
- Precision medicine uses genetic information to inform clinical treatment decisions and identify
prognostic biomarkers and oncogenic drivers4,7,9 - Precision medicine is meant to help improve diagnosis, treatment, and outcomes for patients
through analysis of genomic information10 - Research has shown that better clinical outcomes may result from identifying actionable genetic
alterations and treating with matched therapy11 - Don’t wait to test for FGFR genetic alterations—they may be oncogenic drivers that are actionable12
FGFR testing
Genetic testing options are available that use solid or liquid samples—test for FGFR individually or as part of a broader molecular panel
- Molecular testing is covered by many insurers and Medicare13
- Your pathology team may be able to help determine:
- The best testing methodology for the situation
- Which individual tests or panels are appropriate
Are you testing your patients for FGFR genetic alterations?
There are several types of genetic tests for FGFR alterations, including9:
 Next-generation sequencing (NGS) broadly detects DNA mutations, copy number variations, and gene fusions
Next-generation sequencing (NGS) broadly detects DNA mutations, copy number variations, and gene fusions
- NGS can be performed on a range of cancer types using solid tissue, blood, and bone marrow samples
- Blood samples are appropriate in specific clinical circumstances, and should be followed up with tissue-based analysis if no oncogenic driver is identified
 Polymerase chain reaction (PCR) amplifies one or more copies of a DNA sequence to allow for detection and analysis of genetic mutations
Polymerase chain reaction (PCR) amplifies one or more copies of a DNA sequence to allow for detection and analysis of genetic mutations
- PCR is performed using a tissue sample
- PCR is an option in certain situations to identify a subset of variants
Considerations when selecting NGS or PCR for molecular screening
NGS
- No need for prior DNA sequence knowledge14
- Analyze multiple variants across multiple
genes with a single test15 - Larger amount of tissue may be needed16
- Turnaround time: hours to days17
- Higher overall cost17
PCR
- Only known genes can be tested18
- Analyze a specific DNA or RNA fragment18
- Trace amount of DNA or RNA needed18
- Turnaround time: hours17
- Lower overall cost17
NGS molecular profiling is becoming standard practice for many patients with advanced disease9
To see a list of companion diagnostics approved by the U.S. FDA, visit www.fda.gov/companiondiagnostics.
FGFR testing labs

Check with your pathology team about molecular testing options. If your clinical practice is part of an integrated delivery network or an academic institution, consult with your internal laboratory.
Use Proprietary Laboratory Analyses (PLA) Code 054U for the PCR tissue FGFR test.
Reach out to your reference laboratory for CPT® (Current Procedural Terminology) code information.
Consider ordering reflex testing for FGFR alterations at diagnosis
Reflex testing, adding lab tests to obtain additional information, including genetic alterations, may be a good option
for your patients with solid tumors. Consider ordering testing for FGFR genetic alterations at diagnosis and/or at progression.19,20
‡Reference laboratories shown above may not be a complete list; please check with your reference laboratory to see if they can run an FGFR test.